Technical Report 234, c4e-Preprint Series, Cambridge
Reactivity of polycyclic aromatic hydrocarbon radicals: implications for soot formation
Reference: Technical Report 234, c4e-Preprint Series, Cambridge, 2019
Associated Themes:
Highlights
- The reactivity of aromatic soot precursors are studied.
- Covalent bond energies between aromatic soot precursors are calculated.
- Molecular structures combining physical interactions and covalent bonds are shown.
Abstract
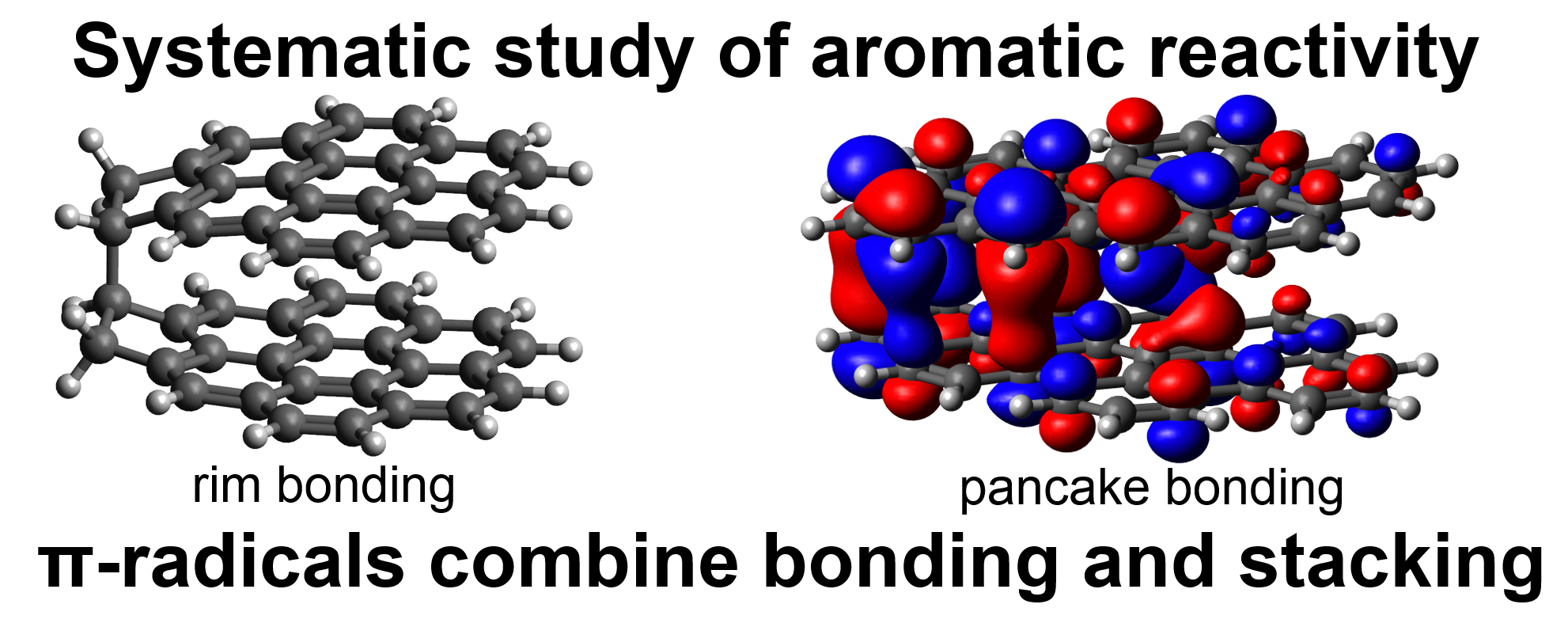 This paper presents a systematic study of the reactivity of polycyclic aromatic hydrocarbons (PAH), identifying crosslinks that permit the combination of physical π-stacking interactions and covalent bonding. Dispersion corrected hybrid density functional theory was used to identify the location of reactive sites on PAHs using the average local ionisation potential. The bond energies formed between these various reactive sites were then computed. σ-radicals were found to be the most reactive, forming bonds with other radicals and some reactive closed shell edge types. Partially saturated rim-based pentagonal rings were found to form localised π-radicals with high reactivity. This site, in addition to resonantly stabilised π-radicals, was found to be capable of bonding and stacking, which is explored for a variety of larger species. Localised π-radicals, in particular, were found to form strongly bound stacked complexes indicating a potentially important role in soot formation.
This paper presents a systematic study of the reactivity of polycyclic aromatic hydrocarbons (PAH), identifying crosslinks that permit the combination of physical π-stacking interactions and covalent bonding. Dispersion corrected hybrid density functional theory was used to identify the location of reactive sites on PAHs using the average local ionisation potential. The bond energies formed between these various reactive sites were then computed. σ-radicals were found to be the most reactive, forming bonds with other radicals and some reactive closed shell edge types. Partially saturated rim-based pentagonal rings were found to form localised π-radicals with high reactivity. This site, in addition to resonantly stabilised π-radicals, was found to be capable of bonding and stacking, which is explored for a variety of larger species. Localised π-radicals, in particular, were found to form strongly bound stacked complexes indicating a potentially important role in soot formation.
Material from this preprint has been published in The Journal of Physical Chemistry C.




