π-Diradical aromatic soot precursors in flames
- High resolution atomic force microscopy revealed species with localised π-monoradicals and π-diradicals
- Thermally stable bonds were formed between diradicals enabling barrierless chain reactions to proceed
- Collisons between π-diradicals were studied with quantum mechanical/molecular mechanics and internal rotors were found to enhance reaction efficiencies
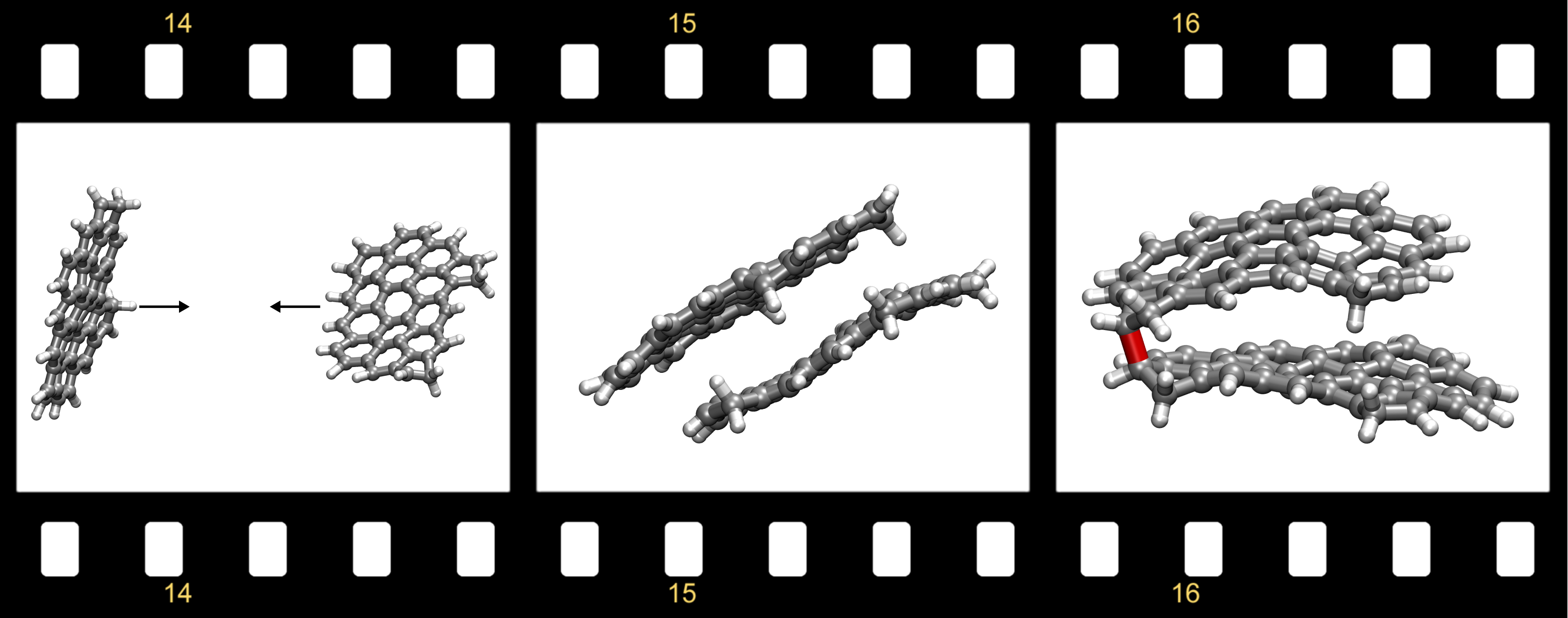 Soot emitted from incomplete combustion of hydrocarbon fuels contributes to global warming and causes human disease. The mechanism by which soot nanoparticles form within hydrocarbon flames is still an unsolved problem in combustion science. Mechanisms proposed to date involving purely chemical growth are limited by slow reaction rates, whilst mechanisms relying on solely physical interactions between molecules are limited by weak intermolecular interactions that are unstable at flame temperatures. Here we show evidence for a reactive diradical aromatic soot precursor imaged using non-contact atomic force microscopy. Localisation of π-electrons on non-hexagonal rings was found to allow for Kekulé aromatic soot precursors to possess a triplet diradical ground states. Barrierless chain reactions are shown between these reactive sites, which provide thermally stable aromatic rim-linked hydrocarbons under flame conditions. Quantum molecular dynamics simulations demonstrate physical condensation of aromatics that survive for tens of picoseconds. Bound internal rotors then enable the reactive sites to find each other and become chemically crosslinked before dissociation. These species provide a rapid, thermally stable chain reaction toward soot nanoparticle formation and could provide molecular targets for limiting the emission of these toxic combustion products.
Soot emitted from incomplete combustion of hydrocarbon fuels contributes to global warming and causes human disease. The mechanism by which soot nanoparticles form within hydrocarbon flames is still an unsolved problem in combustion science. Mechanisms proposed to date involving purely chemical growth are limited by slow reaction rates, whilst mechanisms relying on solely physical interactions between molecules are limited by weak intermolecular interactions that are unstable at flame temperatures. Here we show evidence for a reactive diradical aromatic soot precursor imaged using non-contact atomic force microscopy. Localisation of π-electrons on non-hexagonal rings was found to allow for Kekulé aromatic soot precursors to possess a triplet diradical ground states. Barrierless chain reactions are shown between these reactive sites, which provide thermally stable aromatic rim-linked hydrocarbons under flame conditions. Quantum molecular dynamics simulations demonstrate physical condensation of aromatics that survive for tens of picoseconds. Bound internal rotors then enable the reactive sites to find each other and become chemically crosslinked before dissociation. These species provide a rapid, thermally stable chain reaction toward soot nanoparticle formation and could provide molecular targets for limiting the emission of these toxic combustion products.
- This paper draws from preprint 271: Diradical aromatic soot precursors in flames
- Access the article at the publisher: DOI: 10.1021/jacs.1c05030



