Technical Report 190, c4e-Preprint Series, Cambridge
Vapour pressure and vaporization heat of molecules able to dimerize
Reference: Technical Report 190, c4e-Preprint Series, Cambridge, 2017
- A model of vapour-liquid 1-component equilibrium with vapour dimerization.
- Predicts analytically vapour pressure, vaporization heat and dimer fraction vs. T.
- No fitting - input is 6 standard handbook thermodynamic parameters only.
- Reversely, accurate thermodynamic parameters obtained from vapour pressure data.
- High precision for fuel components under cylinder conditions.
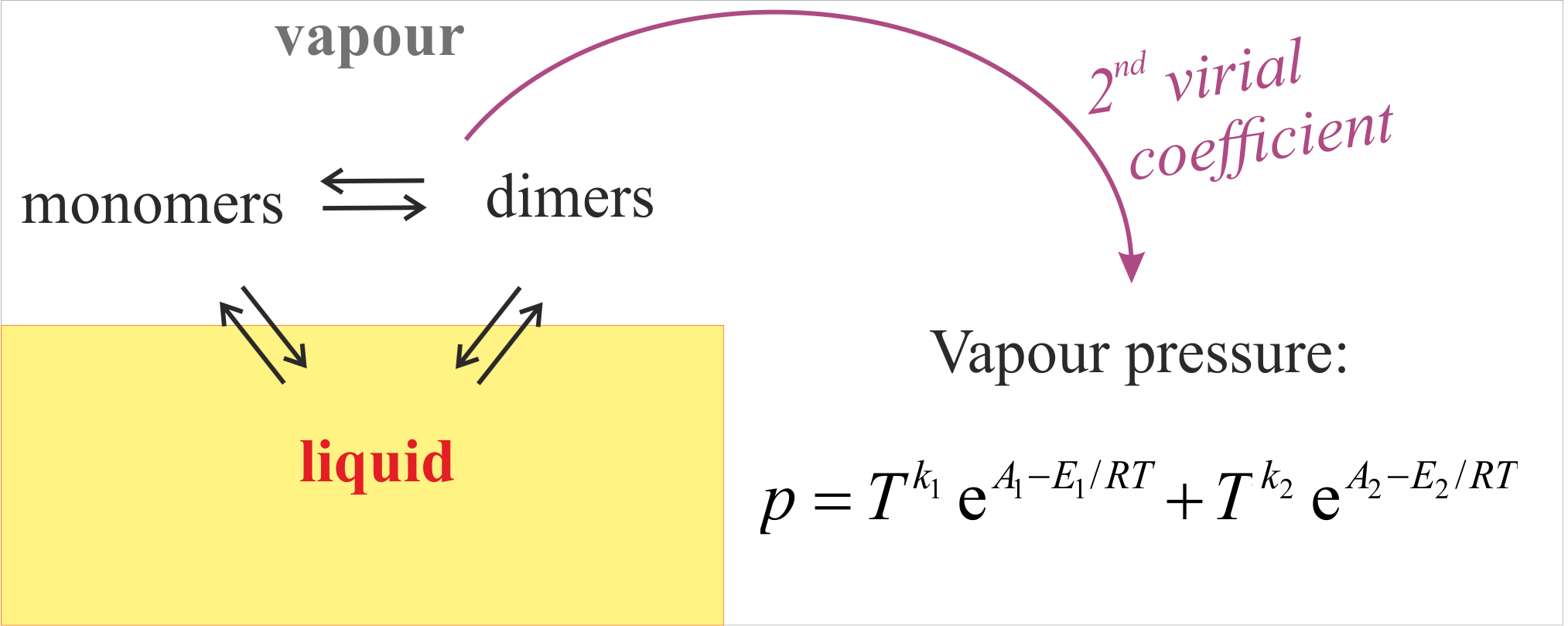 A model of the temperature dependence of the vapour pressure and the heat of vaporization of associated liquids with dimerizing vapours is presented. The result is a simple analytic generalization of the Clapeyron equation, valid with accuracy of 0.1-0.5%, as demonstrated with 8 liquids: formic and acetic acids, methanol, ethanol, water, toluene, heptane and isooctane. It involves only standard handbook parameters: the room temperature vaporization heat and vapour pressure, heat capacities, 2nd virial coefficient, heat of dissociation of the dimers in the gas phase.
A model of the temperature dependence of the vapour pressure and the heat of vaporization of associated liquids with dimerizing vapours is presented. The result is a simple analytic generalization of the Clapeyron equation, valid with accuracy of 0.1-0.5%, as demonstrated with 8 liquids: formic and acetic acids, methanol, ethanol, water, toluene, heptane and isooctane. It involves only standard handbook parameters: the room temperature vaporization heat and vapour pressure, heat capacities, 2nd virial coefficient, heat of dissociation of the dimers in the gas phase.
Material from this preprint has been published in Industrial & Engineering Chemistry Research.
PDF (1.6 MB)



