Reactivity of Polycyclic Aromatic Hydrocarbon Soot Precursors: Kinetics and Equilibria
- Cross-linking rate constants are computed for PAHs with different reactive edges.
- Reactions of σ-radicals and localised π-radicals had the largest rate constants.
- All rate constants are likely too low to explain soot nucleation.
- Equilibrium constants for cross-linking of larger PAHs show enhancement from rim bonding.
- This enhancement is dependent on the reactive edge type and is most noticeable for localised π-radicals.
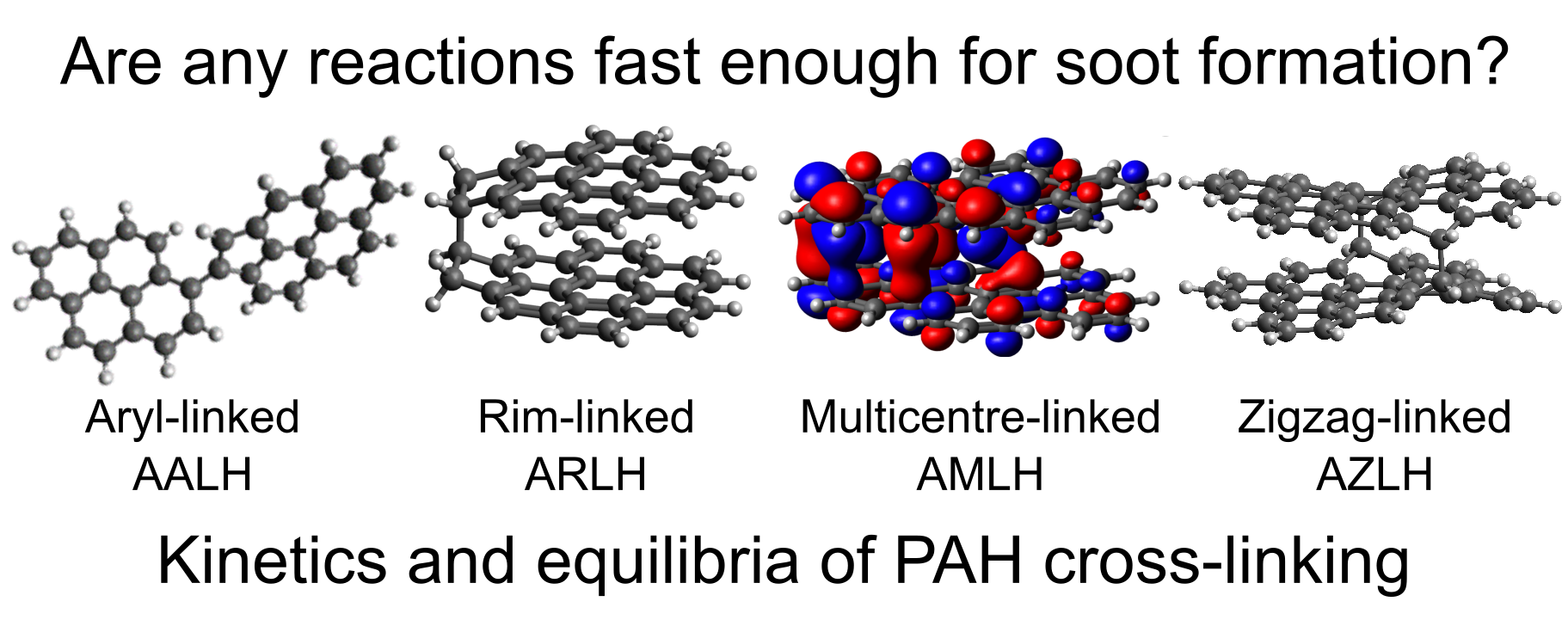 The thermodynamics and kinetics of cross-linking reactions between PAHs of various reactive edge types that are observed in soot precursors are explored using density functional theory. The forward rate constants confirm that reactions involving aryl σ-radicals are faster than others, but rate constants for reactions between aryl σ-radicals and localized π-radicals can be as large or even larger than for two aryl σ-radicals. However, rates for all cross-linking reactions between small PAHs are likely too slow to explain soot formation. The equilibrium constants show that reactions involving σ and π-radical PAHs are the most favorable at flame temperatures. Equilibrium constants for larger PAHs show that the ability to form bonded-and-stacked structures results in enhanced equilibrium constants for the reaction of two large localized π-radicals compared to those for other edge types. This suggests that combined physical and chemical interactions between larger π-radical PAHs could be important in flame environments.
The thermodynamics and kinetics of cross-linking reactions between PAHs of various reactive edge types that are observed in soot precursors are explored using density functional theory. The forward rate constants confirm that reactions involving aryl σ-radicals are faster than others, but rate constants for reactions between aryl σ-radicals and localized π-radicals can be as large or even larger than for two aryl σ-radicals. However, rates for all cross-linking reactions between small PAHs are likely too slow to explain soot formation. The equilibrium constants show that reactions involving σ and π-radical PAHs are the most favorable at flame temperatures. Equilibrium constants for larger PAHs show that the ability to form bonded-and-stacked structures results in enhanced equilibrium constants for the reaction of two large localized π-radicals compared to those for other edge types. This suggests that combined physical and chemical interactions between larger π-radical PAHs could be important in flame environments.
- This paper draws from preprint 258: Reactivity of Polycyclic Aromatic Hydrocarbon Soot Precursors: Kinetics and Equilibria
- Access the article at the publisher: DOI: 10.1021/acs.jpca.0c07811



