The role of NO2 and NO in the mechanism of hydrocarbon degradation leading to carbonaceous deposits in engines
- Isooctane degradation is studied under conditions prevalent at engine cylinder wall.
- NO2 initiates oxidation radical chain in impinged gasoline; >50 products identified.
- O2 alone, in the absence of NOx, does not produce significant liquid fuel degradation.
- Different cPAH sizes disrupt mesophase formation and arrange in a core-shell particle
- If both NOx (NO+NO2) are present, the key reactive intermediates are alkoxy radicals.
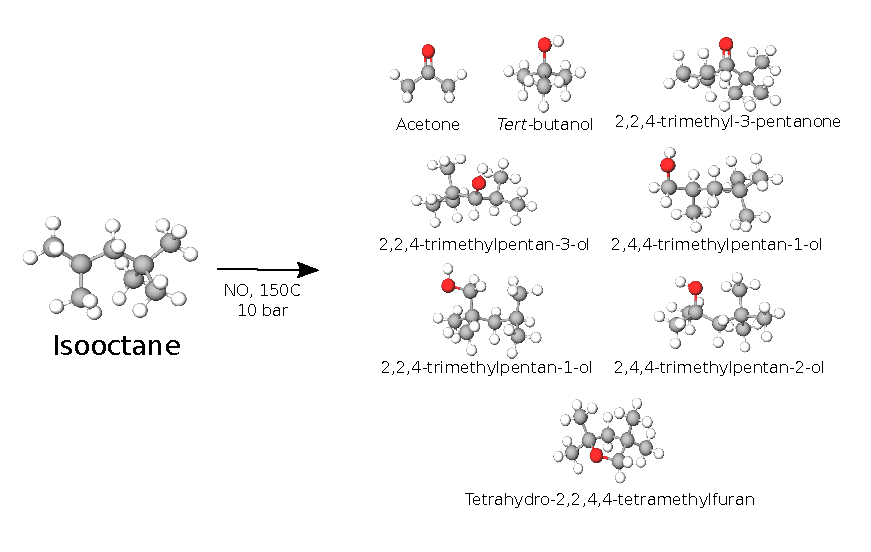 A hypothetical mechanism of degradation of the fuel droplet leaking out from the injector nozzle in a direct injection combustion engine has been proposed recently. This involves as a key step a radical chain oxidation initiated by NO2 and branched by nitric oxide, NO, both produced by the combustion. The degradation causes the formation of injector nozzle carbonaceous deposits. The present work gives an experimental validation of some of the assumptions behind this model. An autoclave is used to oxidize isooctane under conditions relevant to the cylinder wall near the nozzle (~150 °C, 10 bar, 5% O2, 100 ppm of NO2 by mole and 500 ppm NO in the gas phase), and the degradation products are monitored via gas chromatography-mass spectrometry (GC-MS). The results show no observable fuel degradation in the absence of NOx. NO appears to be able to initiate a radical chain by producing NO2. Nitric oxide also alters the radical chain by transforming the alkyl peroxy radicals (ROO⋅) to more reactive alkoxy radicals (RO⋅), resulting in a range of different products. In addition, NO tends to terminate the radical chain by neutralizing a fraction of the alkyl peroxy radicals, producing alkyl nitrates as termination products. The existence of a radical chain is supported by demonstrating the antioxidative action of a radical scavenger. The chemical reaction mechanism is investigated, based on the detected products, and the key species involved in the degradation process are identified.
A hypothetical mechanism of degradation of the fuel droplet leaking out from the injector nozzle in a direct injection combustion engine has been proposed recently. This involves as a key step a radical chain oxidation initiated by NO2 and branched by nitric oxide, NO, both produced by the combustion. The degradation causes the formation of injector nozzle carbonaceous deposits. The present work gives an experimental validation of some of the assumptions behind this model. An autoclave is used to oxidize isooctane under conditions relevant to the cylinder wall near the nozzle (~150 °C, 10 bar, 5% O2, 100 ppm of NO2 by mole and 500 ppm NO in the gas phase), and the degradation products are monitored via gas chromatography-mass spectrometry (GC-MS). The results show no observable fuel degradation in the absence of NOx. NO appears to be able to initiate a radical chain by producing NO2. Nitric oxide also alters the radical chain by transforming the alkyl peroxy radicals (ROO⋅) to more reactive alkoxy radicals (RO⋅), resulting in a range of different products. In addition, NO tends to terminate the radical chain by neutralizing a fraction of the alkyl peroxy radicals, producing alkyl nitrates as termination products. The existence of a radical chain is supported by demonstrating the antioxidative action of a radical scavenger. The chemical reaction mechanism is investigated, based on the detected products, and the key species involved in the degradation process are identified.
- This paper draws from preprint 247: The role of NO2 and NO in the mechanism of hydrocarbon degradation leading to carbonaceous deposits in engines
- Access the article at the publisher: DOI: 10.1016/j.fuel.2020.117218