Skeletal chemical mechanism of high-temperature TEOS oxidation in hydrogen-oxygen environment
- A skeletal mechanism describing TEOS oxidation in flames is proposed.
- A three-stage reduction is used to eliminate unimportant species and reactions.
- Rate parameters for the main TEOS decomposition pathways are refined using transition state theory.
- Energetics of the key reaction channels is improved using CBS-Q method.
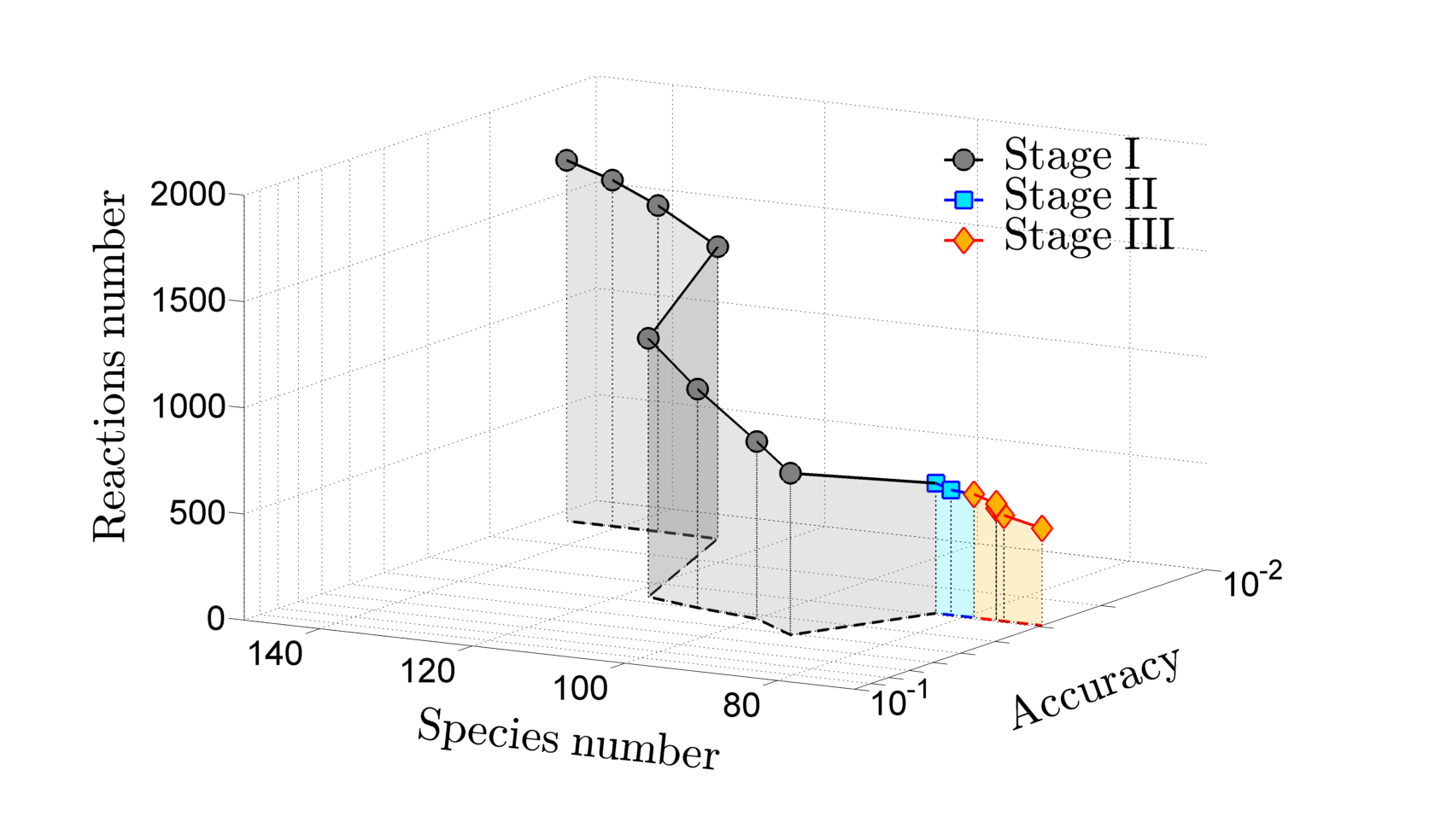 This paper improves the tetraethoxysilane (TEOS) oxidation mechanism proposed by Nurkowski et al. (2015) [17] by refining the rate parameters of the key reaction channels in the mechanism. A skeletal version of the mechanism is proposed for hydrogen–oxygen environment. The rates of ethylene-loss from (tetra-, tri-, di- and dimethyldi-) ethoxysilane are computed using transition state theory. The energetics of the main pathways are refined by performing detailed ab initio calculations using the CBS-Q technique. An analysis of ethanol formation via silicates is also performed resulting in the addition of 27 new silica species to the model. Thermodynamic properties for these species are calculated via the balanced reac- tions method. Reasonably good agreement between the improved model and available experimental data is observed. The subsequent elimination of unimportant species and reactions is achieved via a three- stage reduction procedure. The first and second stages involve the Direct Relation Graph with Error Prop- agation (DRGEP) method, whereas the third stage analyses rate of progress of each reaction. The investi- gated conditions are taken from the experimental studies of TEOS oxidation in oxygen–hydrogen flames. The final skeletal mechanism comprises 70 species and 457 reactions and retains good reproduction of the key model properties across the chosen operating conditions as compared to the full mechanism.
This paper improves the tetraethoxysilane (TEOS) oxidation mechanism proposed by Nurkowski et al. (2015) [17] by refining the rate parameters of the key reaction channels in the mechanism. A skeletal version of the mechanism is proposed for hydrogen–oxygen environment. The rates of ethylene-loss from (tetra-, tri-, di- and dimethyldi-) ethoxysilane are computed using transition state theory. The energetics of the main pathways are refined by performing detailed ab initio calculations using the CBS-Q technique. An analysis of ethanol formation via silicates is also performed resulting in the addition of 27 new silica species to the model. Thermodynamic properties for these species are calculated via the balanced reac- tions method. Reasonably good agreement between the improved model and available experimental data is observed. The subsequent elimination of unimportant species and reactions is achieved via a three- stage reduction procedure. The first and second stages involve the Direct Relation Graph with Error Prop- agation (DRGEP) method, whereas the third stage analyses rate of progress of each reaction. The investi- gated conditions are taken from the experimental studies of TEOS oxidation in oxygen–hydrogen flames. The final skeletal mechanism comprises 70 species and 457 reactions and retains good reproduction of the key model properties across the chosen operating conditions as compared to the full mechanism.
- This paper draws from preprint 160: Skeletal chemical mechanism of high-temperature TEOS oxidation in hydrogen-oxygen environment.
- Access the article at the publisher: DOI: 10.1016/j.combustflame.2016.01.025



