First-Principles Thermochemistry for the Thermal Decomposition of Titanium Tetraisopropoxide
- First-principles calculations used to propose set of TTIP decomposition products.
- Internal rotations are important. Free rotor approximation is good compromise.
- Equilibrium composition analysis performed under typical combustion conditions.
- Ti-OH and O=Ti double bond species are important, in particular Ti(OH)4.
- Carbon-containing Ti-species observed at low temperatures and high TTIP precursor levels.
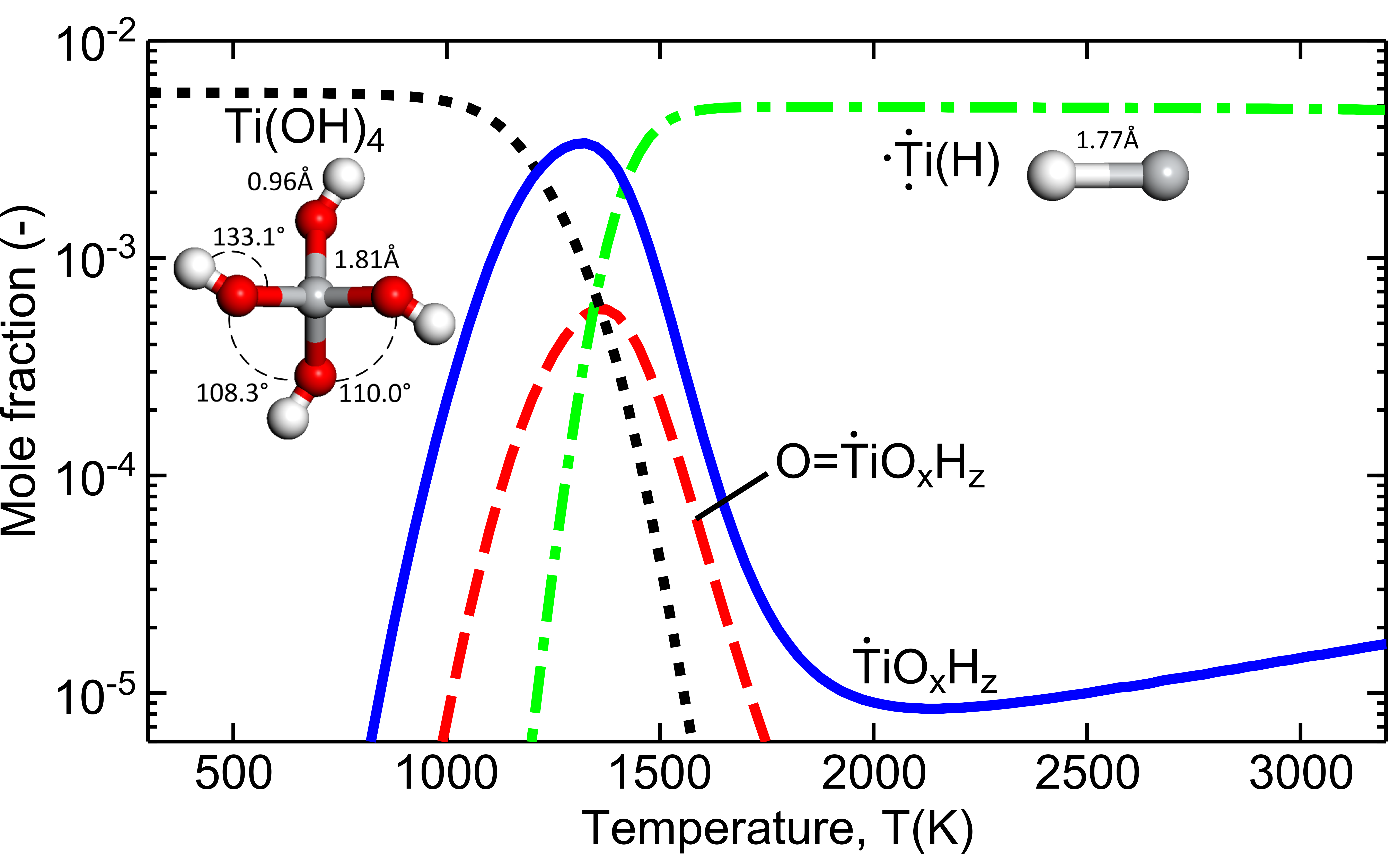 The thermal decomposition of titanium tetraisopropoxide (TTIP) is investigated using quantum chemistry, statistical thermodynamics, and equilibrium composition analysis. A set of 981 Ti-containing candidate species are proposed systematically on the basis of the thermal breakage of bonds within a TTIP molecule. The ground state geometry, vibrational frequencies and hindrance potentials are calculated for each species at the B97-1/6-311+G(d,p) level of theory. Thermochemical data are computed by applying statistical thermodynamics and, if unknown, the standard enthalpy of formation is estimated using balanced reactions. Equilibrium composition calculations are performed under typical combustion conditions for premixed flames. The thermodynamically stable decomposition products for different fuel mixtures are identified. A strong positive correlation is found between the mole fractions of Ti species containing carbon and the TTIP precursor concentration.
The thermal decomposition of titanium tetraisopropoxide (TTIP) is investigated using quantum chemistry, statistical thermodynamics, and equilibrium composition analysis. A set of 981 Ti-containing candidate species are proposed systematically on the basis of the thermal breakage of bonds within a TTIP molecule. The ground state geometry, vibrational frequencies and hindrance potentials are calculated for each species at the B97-1/6-311+G(d,p) level of theory. Thermochemical data are computed by applying statistical thermodynamics and, if unknown, the standard enthalpy of formation is estimated using balanced reactions. Equilibrium composition calculations are performed under typical combustion conditions for premixed flames. The thermodynamically stable decomposition products for different fuel mixtures are identified. A strong positive correlation is found between the mole fractions of Ti species containing carbon and the TTIP precursor concentration.
- This paper draws from preprint 151: First-principles thermochemistry for the thermal decomposition of titanium tetra-isopropoxide
- Access the article at the publisher: DOI: 10.1021/acs.jpca.5b01721



