A density functional theory study on the kinetics of seven-member ring formation in polyaromatic hydrocarbons
- Density Functional Theory was used to compute the process rate constants for seven-member ring formation in PAHs via both HACA and bay closure pathways.
- Kinetic simulations in a 0 Dimensional reactor showed that the dominant pathways are based on hydrogen abstraction, but the carbene pathway becomes important at higher temperatures.
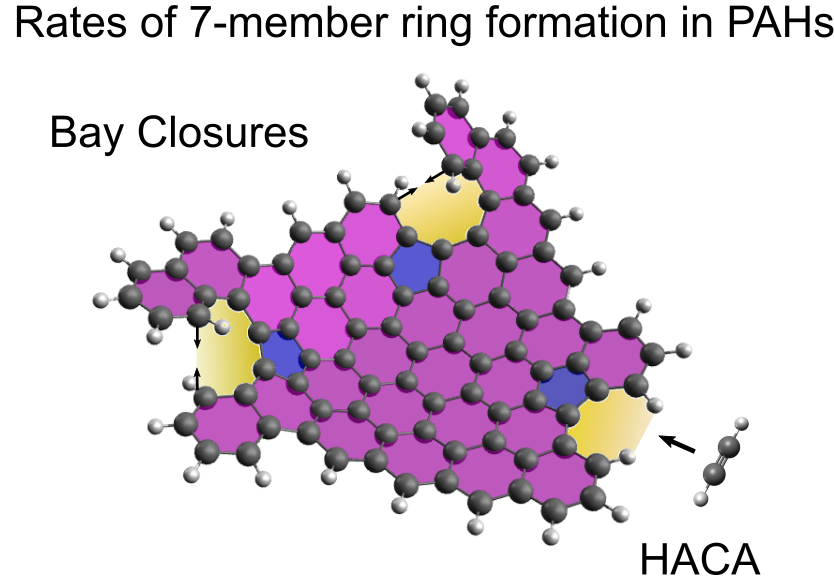 In this work, the kinetics of seven-member ring formation in polycyclic aromatic hydrocarbons (PAHs) containing a five-member ring is studied by density functional theory. The pathways studied include integration of a seven-member ring by the hydrogen-abstraction-acetylene-addition (HACA) mechanism for two different PAHs, one closed shell and one resonance-stabilised-radical (RSR) PAH. The pathways were similar in both cases, but the rate of seven-member ring formation by HACA was seen to be faster for the resonance-stabilised-radical PAH. Formation of a seven member ring by bay closure processes facilitated through hydrogen abstraction, hydrogen addition, carbene formation, and direct cyclisation were also studied for two PAHs. In general, the pathways were rather similar for both PAHs, aside from the direct cyclisation route. The rate constants determined for the pathways were then used in kinetic simulations in 0D homogeneous reactors. The results showed that for the RSR PAH, the initial abstraction site is important, with the seven-member ring mainly being formed when abstraction occurs near the five member ring. This was not the case for the closed shell PAH. Additionally, the RSR PAH product was able to undergo an azulene to naphthalene-like transformation at longer timescales. For the bay closures, it was seen for both PAHs that the hydrogen abstraction facilitated bay closures contributes the most to seven-member ring formation at temperatures up to 2000 K, but for very high temperatures of 2500 K, the carbene route becomes the most important contributor. The formation of seven-member rings occurred within 1 ms for all cases studied in the 0D reactors, suggesting that seven-member ring formation in PAHs containing a five-member ring is possible at flame temperatures.
In this work, the kinetics of seven-member ring formation in polycyclic aromatic hydrocarbons (PAHs) containing a five-member ring is studied by density functional theory. The pathways studied include integration of a seven-member ring by the hydrogen-abstraction-acetylene-addition (HACA) mechanism for two different PAHs, one closed shell and one resonance-stabilised-radical (RSR) PAH. The pathways were similar in both cases, but the rate of seven-member ring formation by HACA was seen to be faster for the resonance-stabilised-radical PAH. Formation of a seven member ring by bay closure processes facilitated through hydrogen abstraction, hydrogen addition, carbene formation, and direct cyclisation were also studied for two PAHs. In general, the pathways were rather similar for both PAHs, aside from the direct cyclisation route. The rate constants determined for the pathways were then used in kinetic simulations in 0D homogeneous reactors. The results showed that for the RSR PAH, the initial abstraction site is important, with the seven-member ring mainly being formed when abstraction occurs near the five member ring. This was not the case for the closed shell PAH. Additionally, the RSR PAH product was able to undergo an azulene to naphthalene-like transformation at longer timescales. For the bay closures, it was seen for both PAHs that the hydrogen abstraction facilitated bay closures contributes the most to seven-member ring formation at temperatures up to 2000 K, but for very high temperatures of 2500 K, the carbene route becomes the most important contributor. The formation of seven-member rings occurred within 1 ms for all cases studied in the 0D reactors, suggesting that seven-member ring formation in PAHs containing a five-member ring is possible at flame temperatures.
- This paper draws from preprint 245: A density functional theory study on the kinetics of seven-member ring formation in polyaromatic hydrocarbons
- Access the article at the publisher: DOI: 10.1016/j.combustflame.2020.03.032



