A systematic method to estimate and validate enthalpies of formation using error-cancelling balanced reactions
- Systematic method to obtain informed estimates of the standard enthalpy of formation.
- Linear programming used to identify error-cancelling balanced reactions.
- Assessment of the consistency of provided reference data.
- Method is applied to test cases from organic and inorganic chemistry, including transition metal complexes.
- Revised reference values of the standard enthalpy of formation for TiOCl and TiO(OH)2.
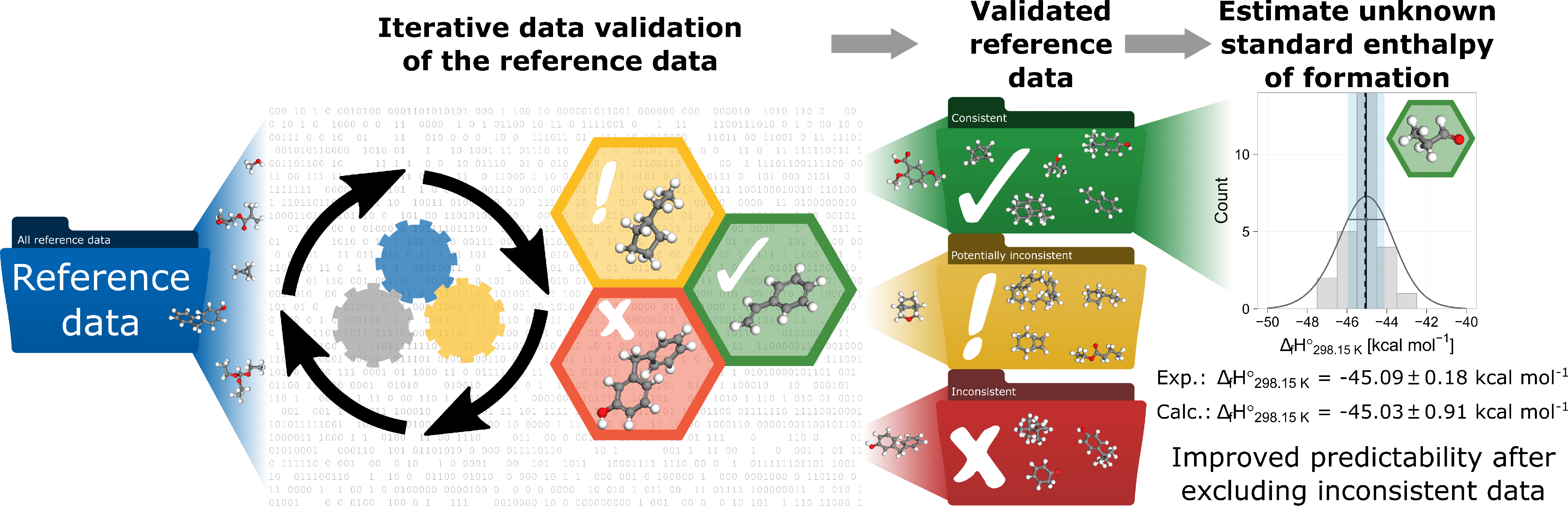 This paper presents an automated framework that uses overlapping subsets of reference data to systematically derive an informed estimate of the standard enthalpy of formation of chemical species and assess the consistency of the reference data. The theory of error-cancelling balanced reactions (EBRs) is used to calculate estimates of the standard enthalpy of formation. Individual EBRs are identified using linear programming. The first part of the framework recursively identifies multiple EBRs for specified target species. A distribution of estimates can then be determined for each species from which an informed estimate of the enthalpy is derived. The second part of the framework iteratively isolates inconsistent reference data and improves the prediction accuracy by excluding such data. The application of the framework is demonstrated for test cases from organic and inorganic chemistry, including transition metal complexes. Its application to a set of 920 carbon, hydrogen and oxygen containing species resulted in a rapid decrease of the mean absolute error for estimates of the enthalpy of formation of each species due to the identification and exclusion of inconsistent reference data. Its application to titanium-containing species identified that the available reference values of TiOCl and TiO(OH)2 are inconsistent and need further attention. Revised values are calculated for both species. A comparison with popular high-level quantum chemistry methods shows that the framework is able to use affordable density functional theory (DFT) calculations to deliver highly accurate estimates of the standard enthalpy of formation, comparable to high-level quantum chemistry methods for both hydrocarbons and transition metal complexes.
This paper presents an automated framework that uses overlapping subsets of reference data to systematically derive an informed estimate of the standard enthalpy of formation of chemical species and assess the consistency of the reference data. The theory of error-cancelling balanced reactions (EBRs) is used to calculate estimates of the standard enthalpy of formation. Individual EBRs are identified using linear programming. The first part of the framework recursively identifies multiple EBRs for specified target species. A distribution of estimates can then be determined for each species from which an informed estimate of the enthalpy is derived. The second part of the framework iteratively isolates inconsistent reference data and improves the prediction accuracy by excluding such data. The application of the framework is demonstrated for test cases from organic and inorganic chemistry, including transition metal complexes. Its application to a set of 920 carbon, hydrogen and oxygen containing species resulted in a rapid decrease of the mean absolute error for estimates of the enthalpy of formation of each species due to the identification and exclusion of inconsistent reference data. Its application to titanium-containing species identified that the available reference values of TiOCl and TiO(OH)2 are inconsistent and need further attention. Revised values are calculated for both species. A comparison with popular high-level quantum chemistry methods shows that the framework is able to use affordable density functional theory (DFT) calculations to deliver highly accurate estimates of the standard enthalpy of formation, comparable to high-level quantum chemistry methods for both hydrocarbons and transition metal complexes.
- This paper draws from preprint 179: A Systematic Method to Estimate and Validate Enthalpies of Formation Using Error-Cancelling Balanced Reactions
- Access the article at the publisher: DOI: 10.1016/j.combustflame.2017.08.013