Phase change of polycyclic aromatic hydrocarbon clusters by mass addition
Highlights
- Molecular Dynamics (MD) simulations of clusters of Polycyclic Aromatic Hydrocarbons (PAHs) are carried out.
- Liquid PAH clusters are characterised by irregular arrangements of its constituent molecules.
- In solid PAH clusters, the molecules tend to be arranged in columns or stacks.
- The melting point of a PAH cluster decreases linearly with the reciprocal of its diameter.
- The Marie QA system is extended to invoke semantic agents to answer questions.
Abstract
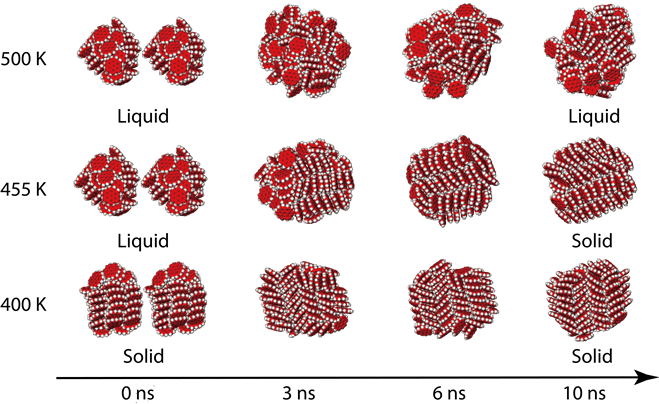 We report the first numerical evidence of the phase change of polycyclic aromatic hydrocarbon (PAH) clusters induced by mass addition using molecular dynamics (MD) simulations. We find that an irregular spherical coronene50 cluster in the liquid phase is transformed to a columnar particle in the solid phase by coalescing with an identical cluster at 455 K. The evolution of the intermolecular energy and the Lindemann Index are used to monitor the phase change process. The physical reason behind this transformation is the size-dependence of the melting point. We hypothesise that this transformation is a possible mechanism explaining the transition from liquid-phase coalescence to solid-phase fractal growth of soot particles.
We report the first numerical evidence of the phase change of polycyclic aromatic hydrocarbon (PAH) clusters induced by mass addition using molecular dynamics (MD) simulations. We find that an irregular spherical coronene50 cluster in the liquid phase is transformed to a columnar particle in the solid phase by coalescing with an identical cluster at 455 K. The evolution of the intermolecular energy and the Lindemann Index are used to monitor the phase change process. The physical reason behind this transformation is the size-dependence of the melting point. We hypothesise that this transformation is a possible mechanism explaining the transition from liquid-phase coalescence to solid-phase fractal growth of soot particles.
Access options
- This paper draws from preprint 131: Phase change of polycyclic aromatic hydrocarbon clusters by mass addition
- Access the article at the publisher: DOI: 10.1016/j.carbon.2014.04.089



