Microkinetic Modeling of Fischer-Tropsch Synthesis over Cobalt Catalysts
- A detailed kinetic mechanism for Fischer-Tropsch synthesis is presented.
- Carberry batch reactor experiments are carried out for a range of hydrogen/carbon monoxide ratios and temperatures.
- A two-stage parameter estimation is carried out to determine Arrhenius pre-exponential factors and activation energies.
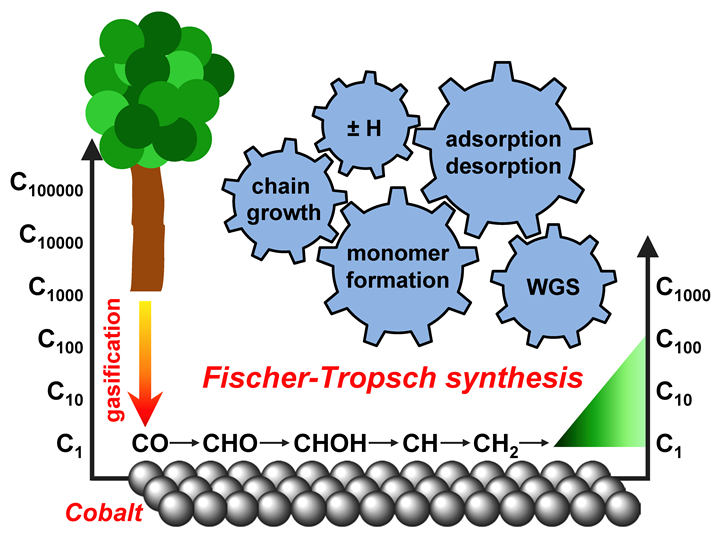 We present a detailed microkinetic analysis of Fischer-Tropsch synthesis on a Co/γ-Al2O3 catalyst over the full range of syngas conversions. The experiments were performed in a Carberry batch reactor with initial H2/CO ratios of between 1.8 and 2.9, temperatures of 469 and 484 K, and an initial pressure of 2 MPa. A reaction mechanism based on the hydrogen-assisted CO activation pathway, which comprises of 128 elementary reactions with 85 free parameters, was compiled to model the experimental results. Each of these elementary reactions belongs to one the following reaction groups: adsorption/desorption, monomer formation, chain growth, hydrogenation/hydrogen abstraction, or water-gas shift. A two-stage parameter estimation method, based on a quasi-random global search followed by a gradient-free local optimization, has been utilized to calculate the values of pre-exponential factors and activation energies. The use of data from batch experiments allowed for an effective analysis of dominating reactions at different stages of syngas conversion.
We present a detailed microkinetic analysis of Fischer-Tropsch synthesis on a Co/γ-Al2O3 catalyst over the full range of syngas conversions. The experiments were performed in a Carberry batch reactor with initial H2/CO ratios of between 1.8 and 2.9, temperatures of 469 and 484 K, and an initial pressure of 2 MPa. A reaction mechanism based on the hydrogen-assisted CO activation pathway, which comprises of 128 elementary reactions with 85 free parameters, was compiled to model the experimental results. Each of these elementary reactions belongs to one the following reaction groups: adsorption/desorption, monomer formation, chain growth, hydrogenation/hydrogen abstraction, or water-gas shift. A two-stage parameter estimation method, based on a quasi-random global search followed by a gradient-free local optimization, has been utilized to calculate the values of pre-exponential factors and activation energies. The use of data from batch experiments allowed for an effective analysis of dominating reactions at different stages of syngas conversion.
- This paper draws from preprint 145: Microkinetic Modeling of Fischer-Tropsch Synthesis over Cobalt Catalysts
- Access the article at the publisher: DOI: 10.1002/cctc.201402662



