Technical Report 289, c4e-Preprint Series, Cambridge
Modelling a detailed kinetic mechanism for electrocatalytic reduction of CO2
Reference: Technical Report 289, c4e-Preprint Series, Cambridge, 2022
- First fully reversible kinetic model towards multiple products
- Model successfully calibrated for experimental results
- Methodology of creating and calibrating complex electrochemical MKM
- Flux analysis indicating coupling reactions without adsorbed CO
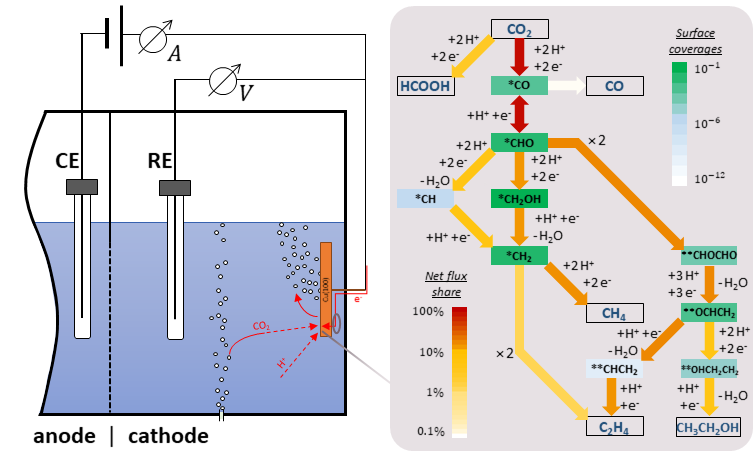 For the first time a fully-elementary reversible kinetic model for electrocatalytic CO2 reduction towards a multitude of different products has been established and verified with experimental data. The detailed reaction mechanism was generated by compiling hypothesized reaction paths and intermediates from many different sources. Thereby a focus was put on distinguishing different embodiments of similar elementary steps: For proton-coupled electron transfer three hydrogenation mechanisms were considered and for intermediates with unclear molecular structure separate
paths were modelled. The micro-kinetic model was fed with tabulated energy parameters and results of Density Functional Theory (DFT) calculations to simulate CO2 reduction on a Cu(100) surface for constant applied potentials. The operating conditions were chosen according to published experimental results in order to compare Faradaic Efficiencies. With these, the model parameters were successfully calibrated across a wide potential range while keeping all values within a tight interval of theoretical bounds derived from ab initio calculations and other theoretical considerations. The calibrated model was found to be in good agreement with the measurement data and also captures qualitative trends of surface coverages reported
for in-situ measurements. Most interestingly, it finds the widely accepted hypothesis of dimerization via *CO intermediates to be inaccurate. Instead, coupling reactions of *CHO and *CH2 intermediates are observed. The shifting of dimerization routes with varying applied potential – especially towards ethylene – is supported by other experimental studies. Furthermore, this work establishes a methodology of creating and calibrating complex electrochemical micro-kinetic models.
For the first time a fully-elementary reversible kinetic model for electrocatalytic CO2 reduction towards a multitude of different products has been established and verified with experimental data. The detailed reaction mechanism was generated by compiling hypothesized reaction paths and intermediates from many different sources. Thereby a focus was put on distinguishing different embodiments of similar elementary steps: For proton-coupled electron transfer three hydrogenation mechanisms were considered and for intermediates with unclear molecular structure separate
paths were modelled. The micro-kinetic model was fed with tabulated energy parameters and results of Density Functional Theory (DFT) calculations to simulate CO2 reduction on a Cu(100) surface for constant applied potentials. The operating conditions were chosen according to published experimental results in order to compare Faradaic Efficiencies. With these, the model parameters were successfully calibrated across a wide potential range while keeping all values within a tight interval of theoretical bounds derived from ab initio calculations and other theoretical considerations. The calibrated model was found to be in good agreement with the measurement data and also captures qualitative trends of surface coverages reported
for in-situ measurements. Most interestingly, it finds the widely accepted hypothesis of dimerization via *CO intermediates to be inaccurate. Instead, coupling reactions of *CHO and *CH2 intermediates are observed. The shifting of dimerization routes with varying applied potential – especially towards ethylene – is supported by other experimental studies. Furthermore, this work establishes a methodology of creating and calibrating complex electrochemical micro-kinetic models.
Material from this preprint has been published in Proceedings of the Combustion Institute.
PDF (2.4 MB)



