Technical Report 137, c4e-Preprint Series, Cambridge
A detailed kinetic study of the thermal decomposition of tetraethoxysilane
Reference: Technical Report 137, c4e-Preprint Series, Cambridge, 2013
- A detailed chemical kinetic mechanism for the thermal decomposition of tetraethoxysilane (TEOS) is proposed.
- A key reaction route is the step-wise four-centre molecular decomposition of TEOS to form silanols and ethylene.
- Another key route is the barrier-less C-C bond cleavage of the ethoxy branches.
- Rate constants for selected reactions were calculated using conventional and variational transition state theories.
- The calculated results are similar to the rate constants of analogous reactions involved in ethanol decomposition.
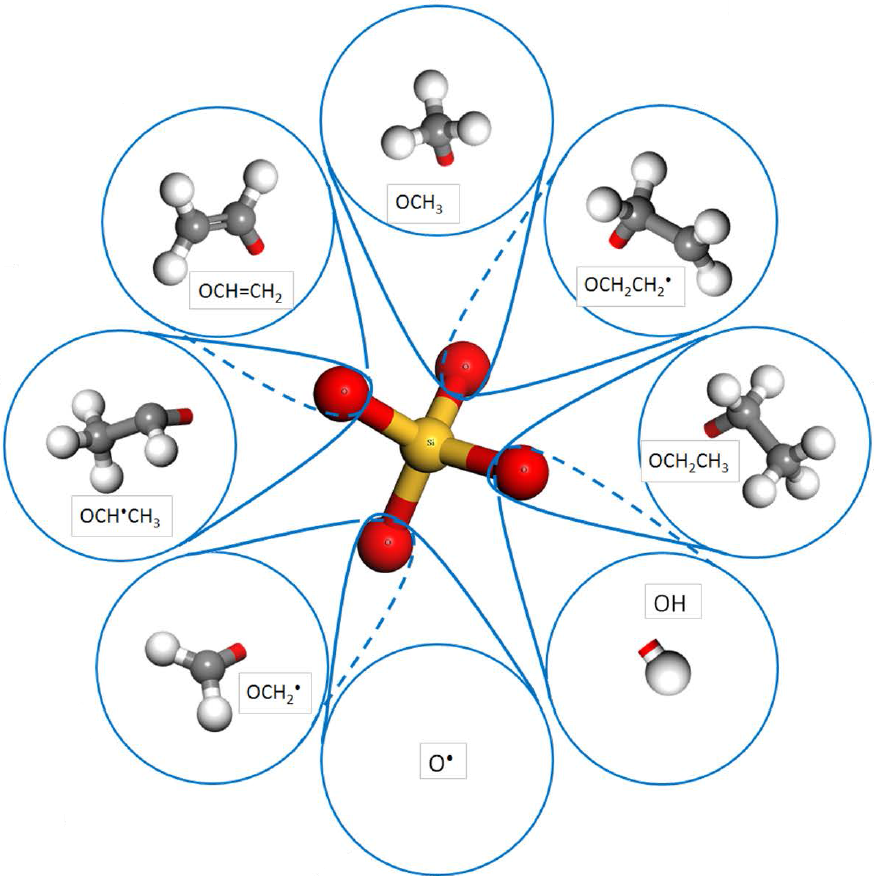 This work presents a detailed kinetic modelling study of the thermal pyrolysis of tetraethoxysilane (TEOS). A chemical mechanism is proposed based on an analogy between the hydrocarbon branches attached to the central silicon atom and an existing mechanism for the decomposition and combustion of ethanol. Important reaction pathways are identified through element flux and sensitivity analyses. It was found that the key reaction routes are the step-wise four-centre molecular decomposition of TEOS to form silanols and ethylene: Si(OH)n(OC2H5)m <=> Si(OH)n+1(OC2H5)m-1 + C2H4 (n + m = 4) and the barrier-less C-C bond cleavage of the ethoxy branches: Si(OH)n(OC2H5)m <=> Si(OH)n(OC2H5)m-1(OCH2) + CH3 (n + m <= 4). Rate constants were calculated using conventional and variational transition state theories (TST and VTST) for all the reactions in the first route and for the methyl radical removal from Si(OH)3(OC2H5) in the second route. The calculated results are similar to the rate constants of the corresponding ethanol reactions, providing support for the analogy with the ethanol decomposition. Simulations using the proposed mechanism are shown to be consistent with experimental data for the decomposition of TEOS.
This work presents a detailed kinetic modelling study of the thermal pyrolysis of tetraethoxysilane (TEOS). A chemical mechanism is proposed based on an analogy between the hydrocarbon branches attached to the central silicon atom and an existing mechanism for the decomposition and combustion of ethanol. Important reaction pathways are identified through element flux and sensitivity analyses. It was found that the key reaction routes are the step-wise four-centre molecular decomposition of TEOS to form silanols and ethylene: Si(OH)n(OC2H5)m <=> Si(OH)n+1(OC2H5)m-1 + C2H4 (n + m = 4) and the barrier-less C-C bond cleavage of the ethoxy branches: Si(OH)n(OC2H5)m <=> Si(OH)n(OC2H5)m-1(OCH2) + CH3 (n + m <= 4). Rate constants were calculated using conventional and variational transition state theories (TST and VTST) for all the reactions in the first route and for the methyl radical removal from Si(OH)3(OC2H5) in the second route. The calculated results are similar to the rate constants of the corresponding ethanol reactions, providing support for the analogy with the ethanol decomposition. Simulations using the proposed mechanism are shown to be consistent with experimental data for the decomposition of TEOS.
Material from this preprint has been published in Proceedings of the Combustion Institute.
PDF (740.2 KB)



