On the role of C4 and C5 products in electrochemical CO2 reduction
- Comprehensive GC-MS study of high-performance eCO2R GDE products
- Identification of over 20 different products, including four- and five-carbon species reported for the first time
- Trends for different isotopes and isomers over applied potential analysed
- Mechanism analysis revealing reduction state before coupling as driving factor for double bond locations and resulting selectivity trends
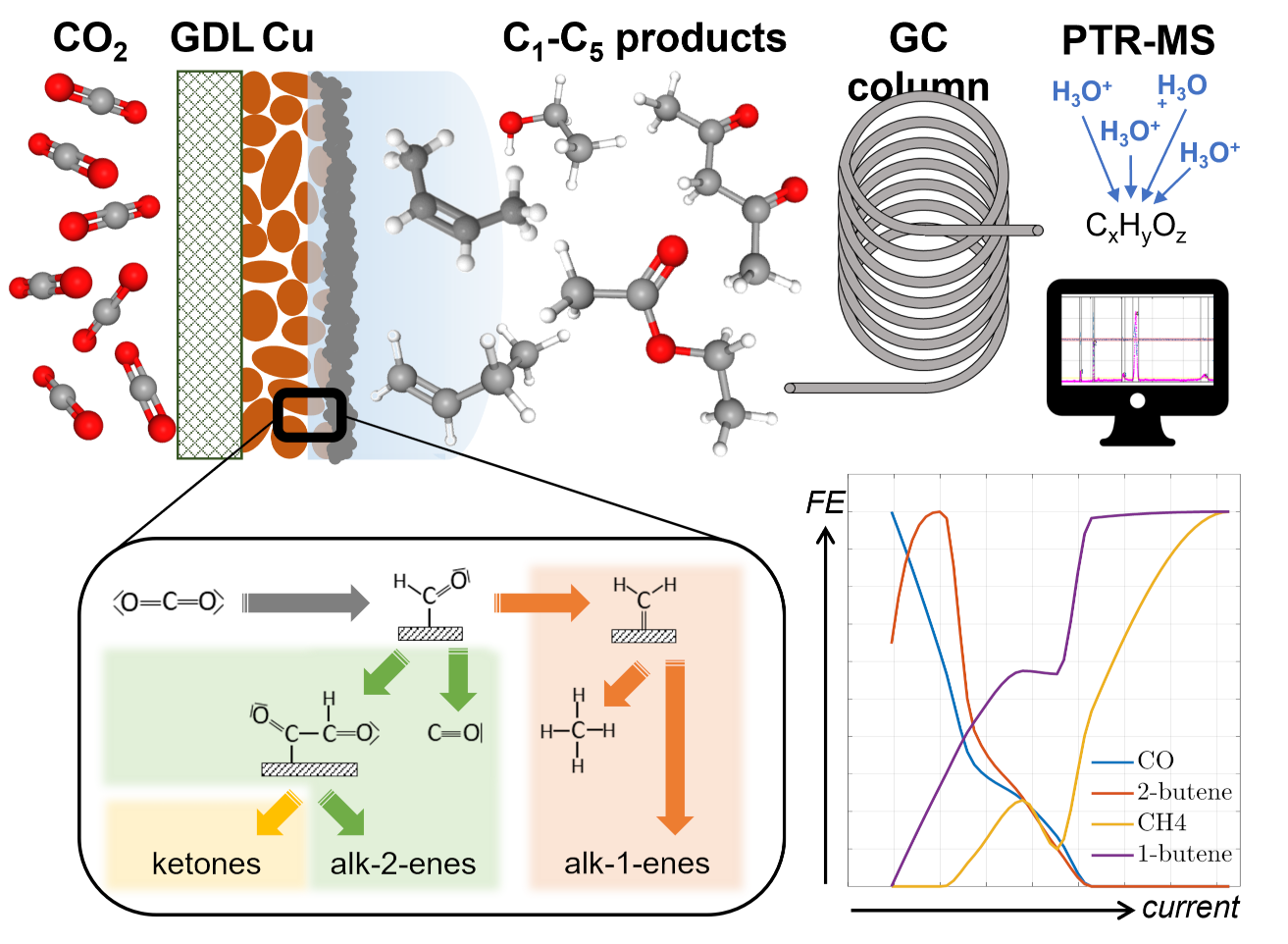 Utilising carbon dioxide by synthesising commodity chemicals via electrocatalysis shows potential for long-term energy storage and industry decarbonisation. The latest copper-based gas-diffusion electrodes can operate at high currents, enabling large conversion rates. However, our incomplete understanding of active reaction paths in this system hinders us from designing catalysts with improved selectivities and reduced poisoning. Here, we identify and analyse ten previously unknown minor products of electrochemical CO2 reduction. Using an ultra-sensitive GC-MS setup, we report more than 20 products, including C5 species for the first time. From the trends in selectivity, we hypothesise two distinct reaction paths: while the coupling of oxygenated intermediates begins at very small potentials and favours double bond formation in the middle of carbon chains, coupling of highly-reduced methane precursors requires a large potential and leads to double bond formation at the chain end. This contribution represents a significant step towards the holistic comprehension of the mechanism for electrocatalytic CO2 reduction and calls for further mechanistic exploration via minor products and investigation of favourable reaction conditions.
Utilising carbon dioxide by synthesising commodity chemicals via electrocatalysis shows potential for long-term energy storage and industry decarbonisation. The latest copper-based gas-diffusion electrodes can operate at high currents, enabling large conversion rates. However, our incomplete understanding of active reaction paths in this system hinders us from designing catalysts with improved selectivities and reduced poisoning. Here, we identify and analyse ten previously unknown minor products of electrochemical CO2 reduction. Using an ultra-sensitive GC-MS setup, we report more than 20 products, including C5 species for the first time. From the trends in selectivity, we hypothesise two distinct reaction paths: while the coupling of oxygenated intermediates begins at very small potentials and favours double bond formation in the middle of carbon chains, coupling of highly-reduced methane precursors requires a large potential and leads to double bond formation at the chain end. This contribution represents a significant step towards the holistic comprehension of the mechanism for electrocatalytic CO2 reduction and calls for further mechanistic exploration via minor products and investigation of favourable reaction conditions.
- This paper draws from preprint 299: On the role of C4 and C5 products in electrochemical CO2 reduction
- Access the article at the publisher: DOI: 10.1039/D2EE03752A



