Preprint 238 published
Preprint 238, "Understanding the anatase-rutile stability in flame-made TiO2", has been published!
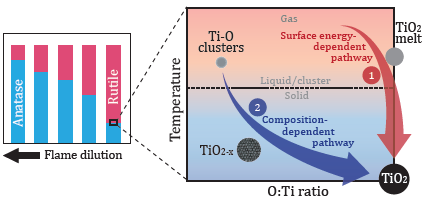
The relative stability of anatase and rutile in stagnation flame synthesis with stoichiometric mixtures is investigated experimentally. The measurements reveal a high sensitivity of anatase-rutile composition to the flame dilution. It is demonstrated that anatase formation is favoured in more dilute (colder) flames while rutile is favoured in less dilute (hotter) flames. A particle model with a detailed description of aggregate morphology and crystal phase composition is applied to investigate the anatase-rutile stability. A size-dependent phase transformation model is implemented in which rutile is formed for particles larger than a "crossover" size while anatase is formed for those smaller. Two formation mechanisms/pathways are discussed and evaluated. In the first pathway, the nascent particles are assumed to be stoichiometric and the crossover size is given by thermodynamic quantities. This hypothesis captures the general trend in the measured anatase-rutile composition but fails to explain the sensitivity. In the second pathway, non-stoichiometric TiO2-x oxide intermediates are assumed and the crossover size is hypothesised to be composition-dependent. This shows an excellent agreement with the experimental data. However, this hypothesis is found to be strongly influenced by assumptions about the initial particle growth stages. This study demonstrates the importance of a better description of the high-temperature chemistry and initial clustering mechanism in order to understand the crystal phase formation.



